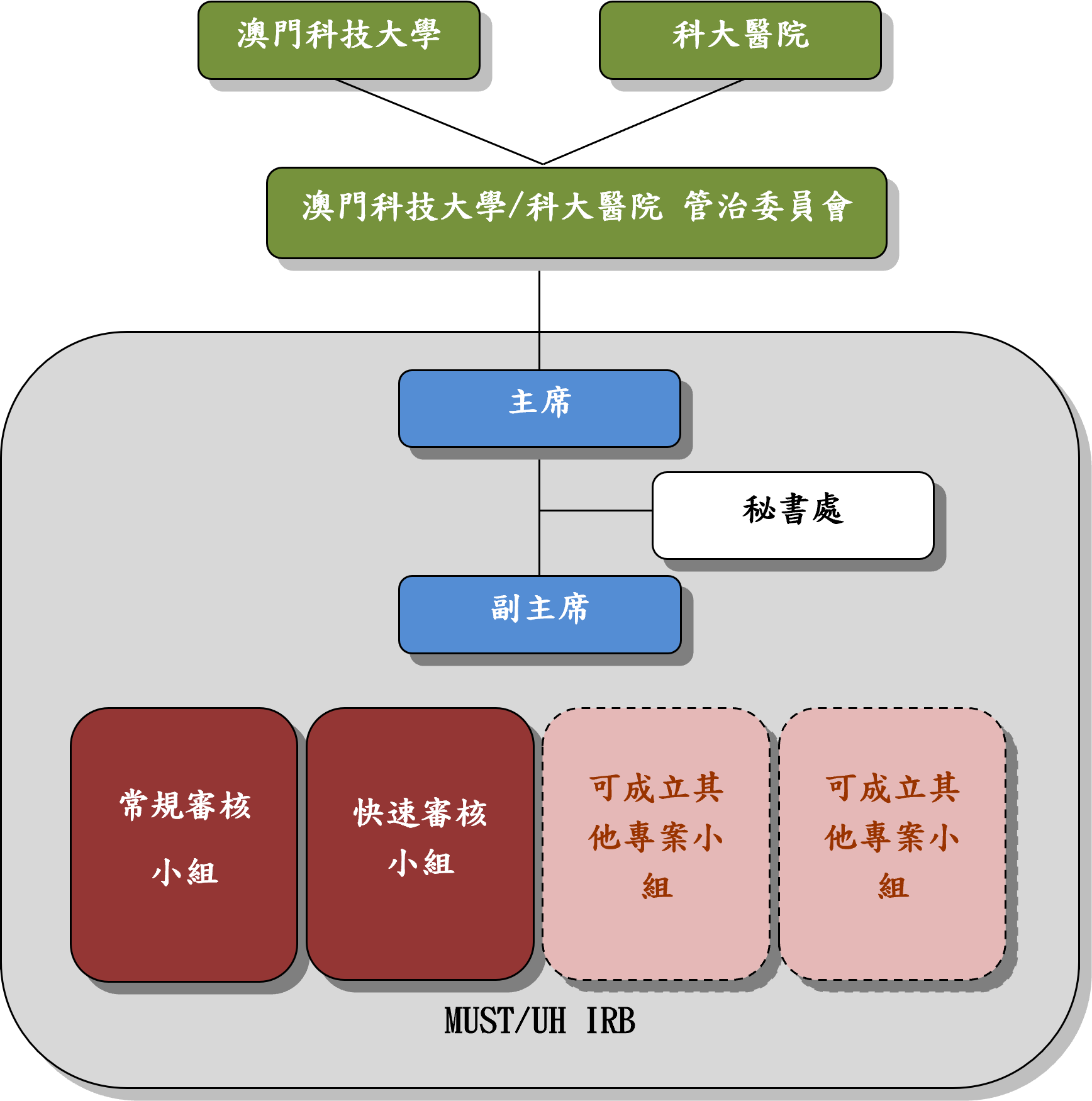
At MUST/UH, clinical studies are overseen by Macau University of Science and Technology / University Hospital Clinical Research Institutional Review Board (MUST/UH IRB).
MUST/UH IRB is an independent committee, which was established in collaboration between MUST and the University Hospital (UH) in accordance with the requirements of the ICH GCP, and is governed by the two organizations through the Governance Committee (GC). It is composed of a diverse group of members, including clinical and medical expertise, non-scientific members whose primary expertise is not in any medical-related area, and independent members with no ties to either MUST or UH. Its primary goal is to protect the rights, safety, and well-being of participants in clinical studies affiliated with MUST and/or UH by providing initial and ongoing ethical and scientific evaluation of the research activities.
MUST/UH IRB operates in compliance with its written SOP and the core tenet of the Declaration of Helsinki and the ICH GCP, as well as other applicable international, national or local ethics standards or regulatory requirements
Macau University of Science and Technology / University Hospital Clinical Research Institutional Review Board (MUST/UH IRB) Secretariat: Stephanie Wong (clinicalresearchirb@must.edu.mo)